40 what is used to label the energy levels of electrons
Electron Energy Level Equations & Examples | What is an Energy Level of ... Energy Levels of an Atom. An atom's electrons do not all exist at the same energy level. While the Bohr model is a great place to start understanding the different energy levels and how they ... The periodic table, electron shells, and orbitals Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. By convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n.
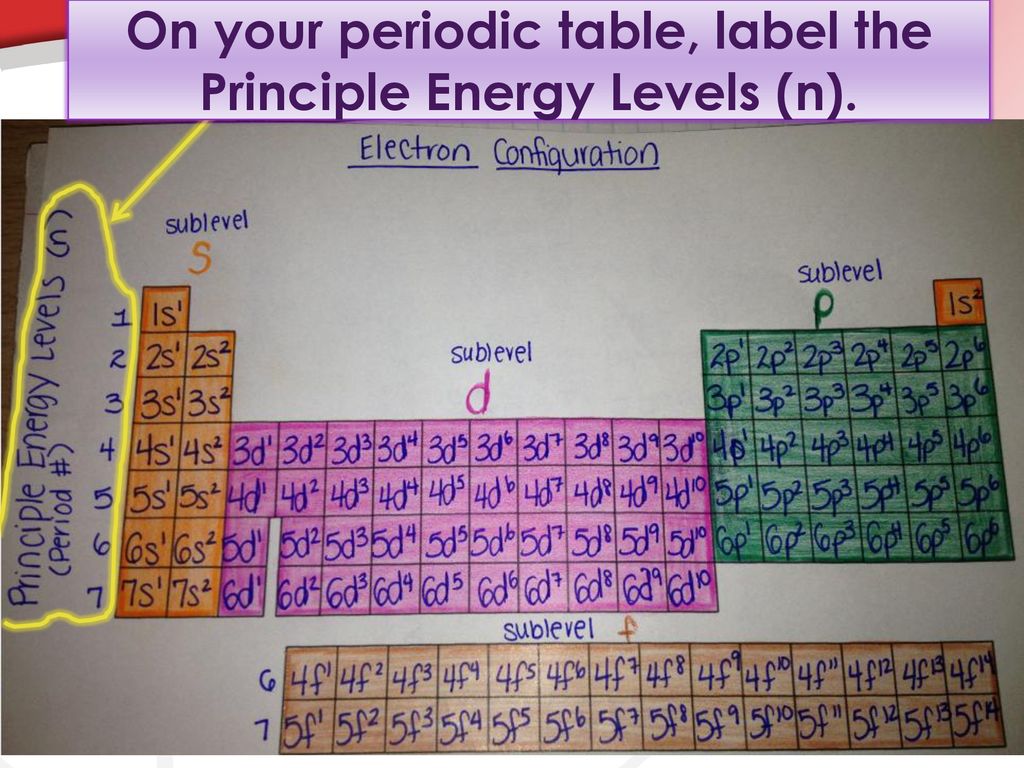
What term is used to label the energy levels of electrons? Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital.
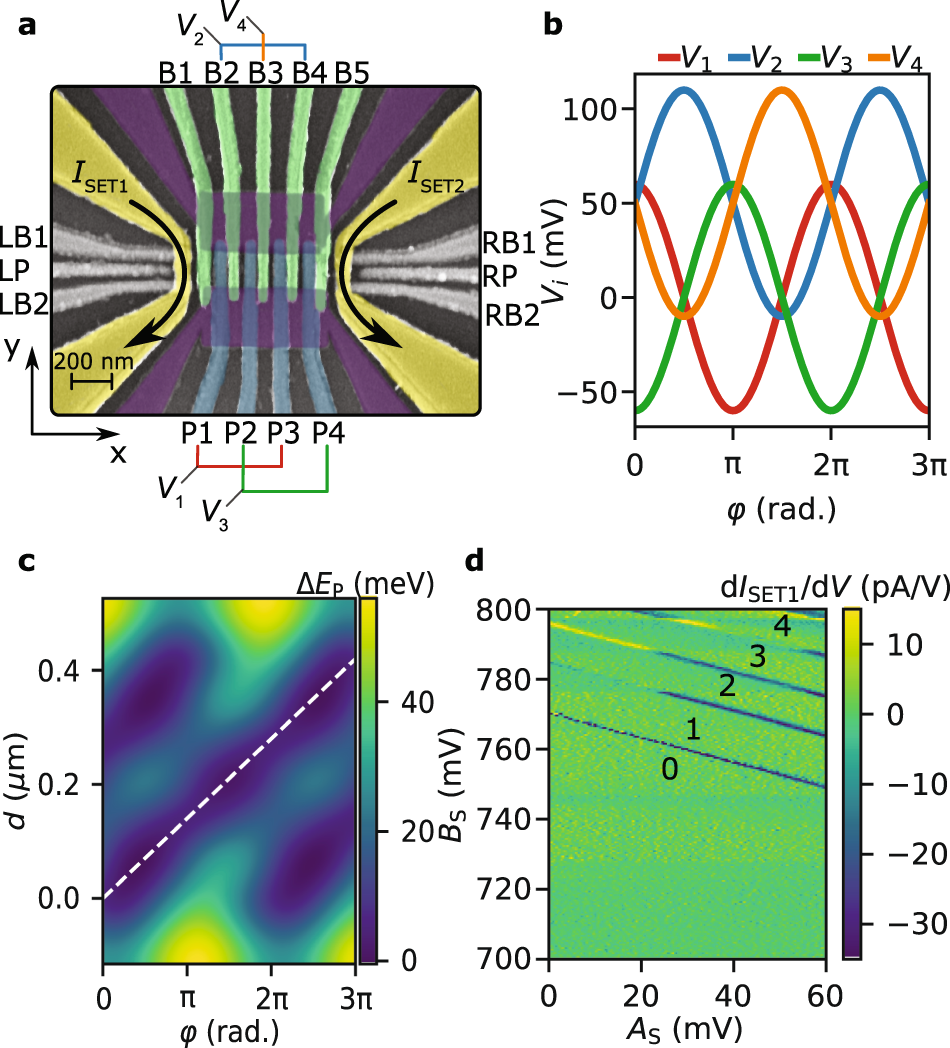
What is used to label the energy levels of electrons
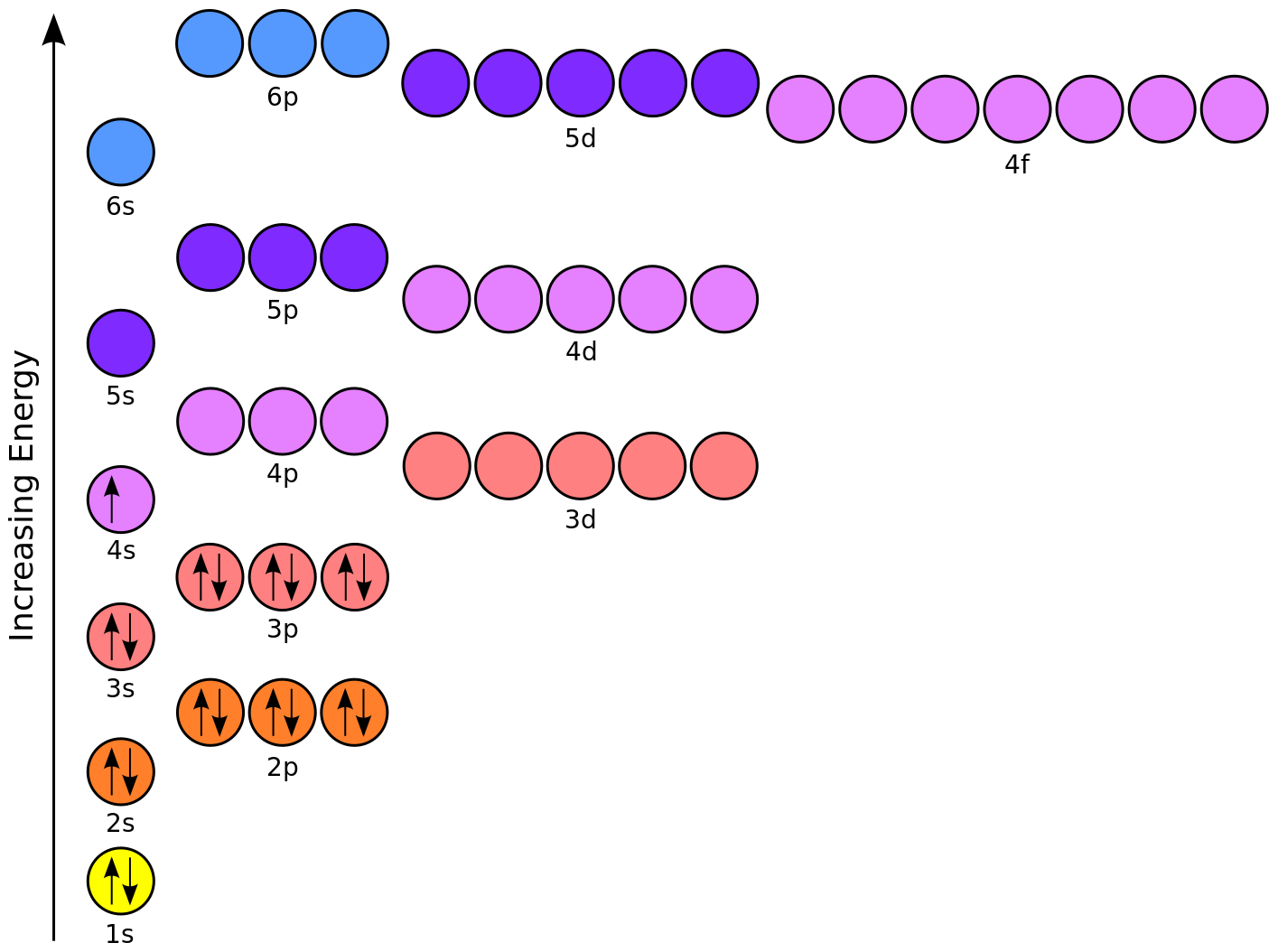
Energy Level ( Read ) | Chemistry Energy levels of atoms, their relation to orbitals, and the significance of electrons at different energy levels. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic. 8.3: Electron Configurations- How Electrons Occupy Orbitals The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are: How to Represent Electrons in an Energy Level Diagram In using the energy level diagram, remember two things: Electrons fill the lowest vacant energy levels first. When there's more than one subshell at a particular energy level, such as at the 3p or 4d levels, only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell.
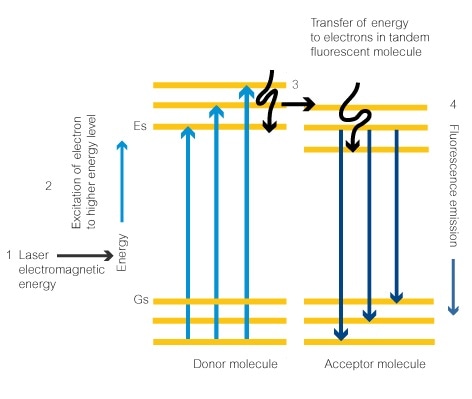
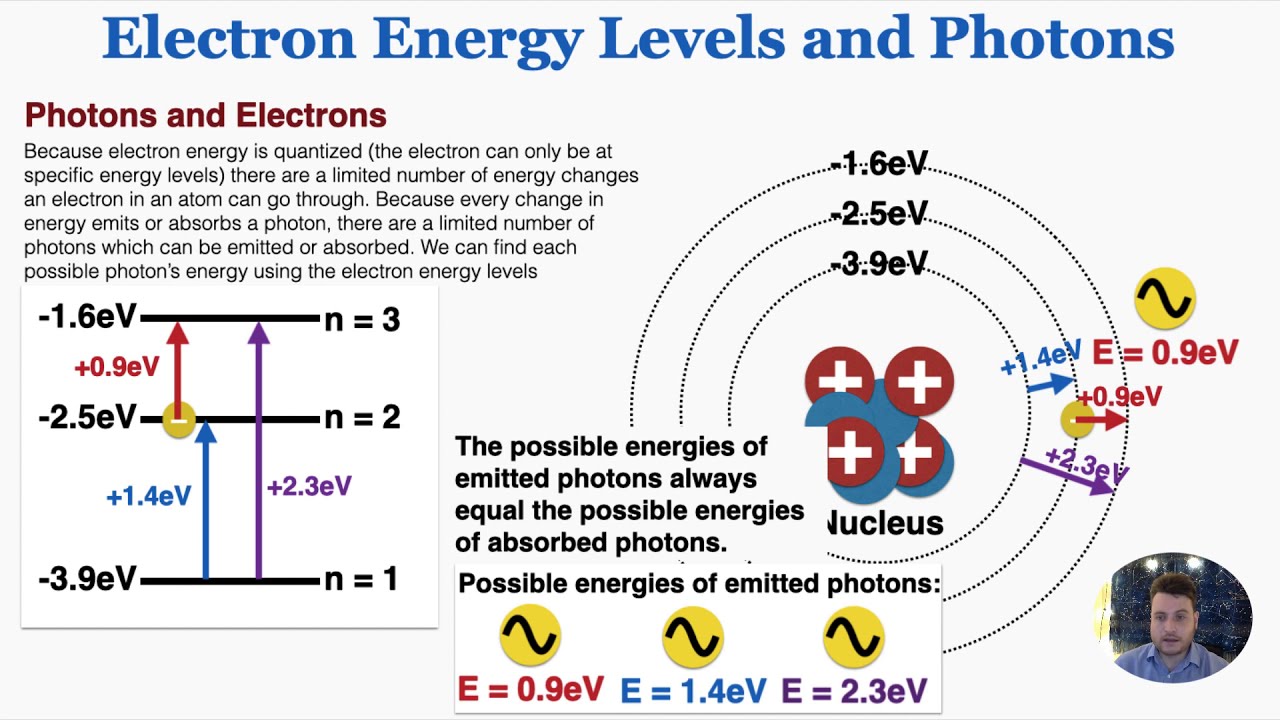
What is used to label the energy levels of electrons. Atomic Energy Levels (video) | Khan Academy The electron can absorb photons that will make it's charge positive, but it will no longer be bound the the atom, and won't be a part of it. For example at -10ev, it can absorb, 4eV (will move to -6eV), 6eV (will move to -4eV), 7eV (will move to -3eV), and anything above 7eV (will leave the atom) 2 comments ( 12 votes) Upvote Downvote Flag more 5.12: Energy Level - Chemistry LibreTexts Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in an atom that move around the positive nucleus at the center. Energy levels are a little like the steps of a staircase. s,p,d,f Orbitals - Chemistry | Socratic The number denotes the energy level of the electron in the orbital. Thus 1 refers to the energy level closest to the nucleus; 2 refers to the next energy level further out, and so on. The letter refers to the shape of the orbital. The letters go in the order s, p, d, f, g, h, i, j, etc. What is the term used to label the energy levels of electrons? The term used to label the energy levels of electrons is called the electron energy levels. The energy levels of electrons are important because they determine how electrons behave. The energy levels of electrons determine how electrons interact with other particles, how they absorb and emit energy, and how they move around.
Solved 7. What is the term used to label the energy levels | Chegg.com Science Chemistry Chemistry questions and answers 7. What is the term used to label the energy levels of electrons? 8. How are s orbitals different from p orbitals? 9. How many electrons can each of the following orbitals hold? a. 2 s=22 d. 6 d=10 b. 3p=146 c. 5f= 10. How many "p" orbitals can there be in any energy level? 11. Circle the letter of the term that is used to label the ener | Quizlet Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals b. quantum mechanical numbers c. quantum d. principal quantum numbers (n) Solutions Verified Solution A Solution B Answered 1 year ago Step 1 1 of 2 The letter of the correct answer is D. Step 2 2 of 2 Atom - Orbits and energy levels In the same way, if energy is added to an atom, an electron can use that energy to make a quantum leap from a lower to a higher orbit. This energy can be supplied in many ways. One common way is for the atom to absorb a photon of just the right frequency.For example, when white light is shone on an atom, it selectively absorbs those frequencies corresponding to the energy differences between ... Energy level A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels.This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can ...
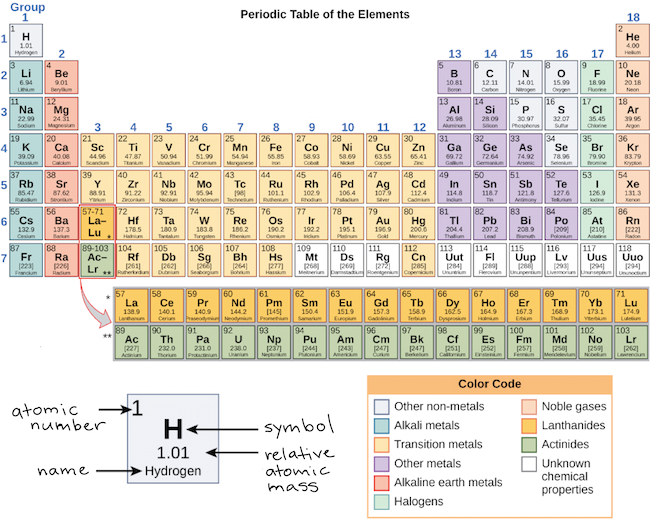
The Periodic Table and Energy-Level Models The electrons surrounding an atom are located in regions around the nucleus called "energy levels". An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first. Chapter 5 Electrons in Atoms Flashcards What is the term that is used to label the energy levels of electrons? principal quantum numbers (𝑛) The letter __________ is used to denote a spherical orbital. S What is the formula for the maximum number of electrons that can occupy a principal energy level? Use 𝑛 for the principal quantum number. 2𝑛² Energy Level and Transition of Electrons Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where n n denotes the principal quantum number: E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n = − n21312 kJ/mol. For a single electron instead of ... Electron Configuration Energy Levels | How to Write Electron ... Labeling the Periodic Table. ... The four energy levels for electrons are s, p, d, and f. s level holds 2 electrons; p level holds 8 electrons; d level holds 10 electrons; f level holds 14 ...
How to Represent Electrons in an Energy Level Diagram In using the energy level diagram, remember two things: Electrons fill the lowest vacant energy levels first. When there's more than one subshell at a particular energy level, such as at the 3p or 4d levels, only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell.
8.3: Electron Configurations- How Electrons Occupy Orbitals The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
Energy Level ( Read ) | Chemistry Energy levels of atoms, their relation to orbitals, and the significance of electrons at different energy levels. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic.



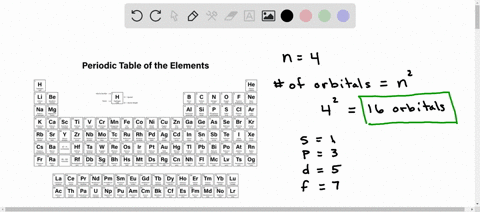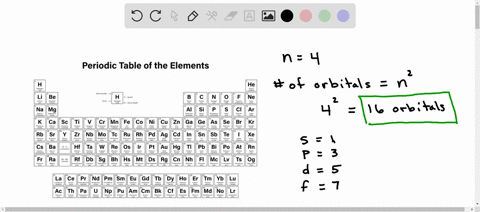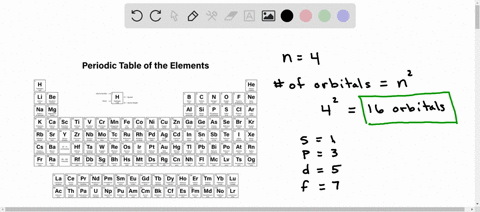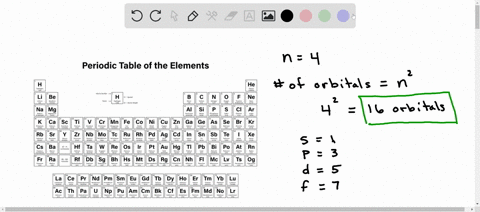

:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)


















Post a Comment for "40 what is used to label the energy levels of electrons"